Europe Medical Device Testing Market Innovation Trends | New Technologies and Future Growth 2025 - 2032
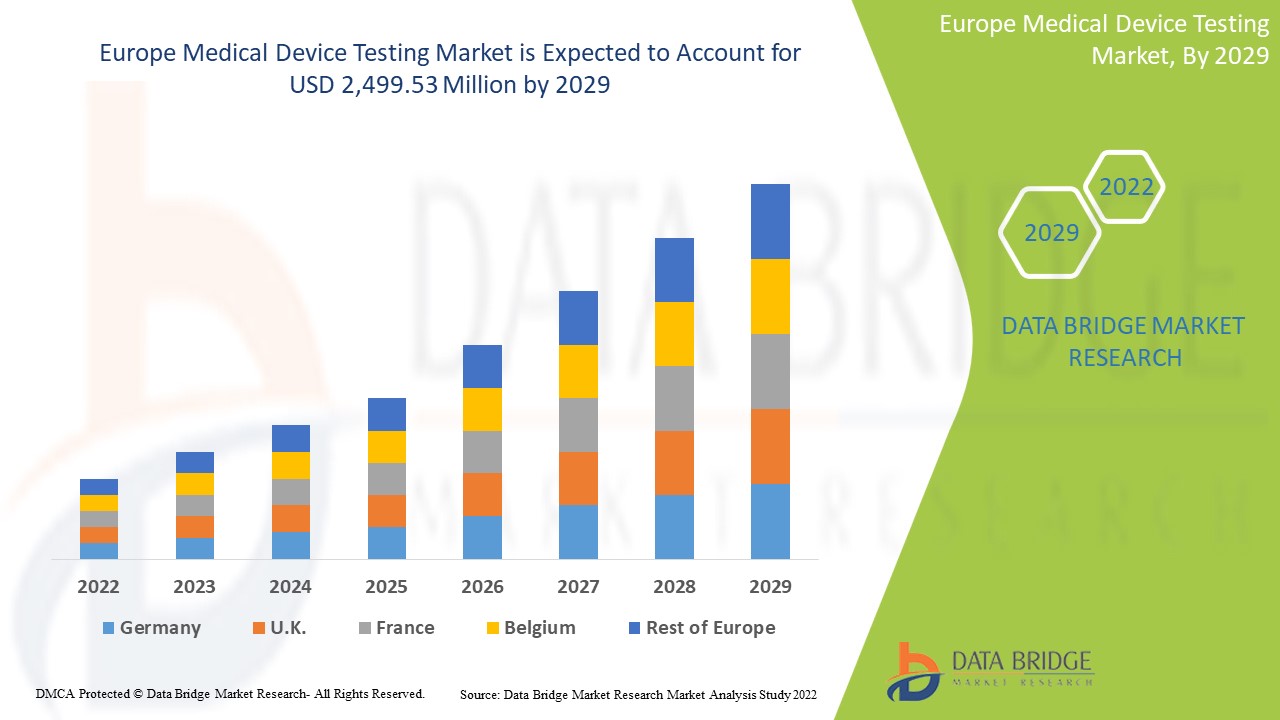 Executive Summary Europe Medical Device Testing Market :
Executive Summary Europe Medical Device Testing Market :
Data Bridge Market Research analyses that the market is growing with a CAGR of 9.8% in the forecast period of 2022 to 2029 and is expected to reach USD 2,499.53 million by 2029 from USD 1,226.46 million in 2021.
The analysis and estimations conducted via the winning Europe Medical Device Testing Market report help to get an idea about the product launches, future products, joint ventures, Market strategy, developments, mergers and acquisitions and effect of the same on sales, Market, promotions, revenue, import, export, and s. The industry analysis report assists in determining and optimizing each stage in the lifecycle of industrial process that includes engagement, acquisition, retention, and monetization. This comprehensive report has estimations of s which are very important for businesses in deciding upon the investment value over the time period. Europe Medical Device Testing Market report examines market drivers, market restraints, challenges, opportunities and key developments in the industry.
The high quality Europe Medical Device Testing Market document contains market insights and analysis for industry which are backed up by SWOT analysis. This report provides a broader perspective of the market place with its comprehensive market insights and analysis which eases surviving and succeeding in the market. Moreover, such market report explains better market perspective in terms of product trends, Market strategy, future products, new geographical markets, future events, sales strategies, customer actions or behaviours. Europe Medical Device Testing Market research report encompasses a far-reaching research on the current conditions of the industry, potential of the market in the present and the future prospects.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Europe Medical Device Testing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/europe-medical-device-testing-market
Europe Medical Device Testing Market Overview
**Segments**
- **By Service Type:** The Europe medical device testing market can be segmented into testing services, inspection services, certification services, and others. Testing services involve the testing of medical devices for quality, safety, and performance. Inspection services focus on assessing the compliance of medical devices with regulatory standards. Certification services involve obtaining certifications to demonstrate that the medical devices meet specified requirements. Other services may include consulting, training, and auditing related to medical device testing.
- **By Sourcing Type:** In terms of sourcing type, the market can be segmented into in-house testing and outsourced testing. In-house testing refers to medical device manufacturers conducting testing within their own facilities. Outsourced testing involves partnering with third-party testing labs or service providers to conduct the necessary testing and evaluation of medical devices.
- **By Technology:** The market can also be segmented based on the technology used for testing. This includes biological safety testing, chemical testing, physical testing, electrical safety testing, and others. Each of these technologies plays a crucial role in ensuring the quality and safety of medical devices in compliance with regulatory standards.
**Market Players**
- **Eurofins Scientific:** Eurofins Scientific is a leading player in the Europe medical device testing market, offering a wide range of testing services for medical devices. The company has a strong reputation for its expertise in regulatory compliance and quality assurance.
- **SGS SA:** SGS SA is another key player in the market, providing comprehensive testing and certification services for medical devices. The company's global presence and extensive experience make it a preferred choice for medical device manufacturers.
- **Intertek Group plc:** Intertek Group plc is a prominent player offering testing, inspection, and certification services for various industries, including the medical device sector. The company's focus on quality and safety standards positions it as a reliable partner for medical device testing.
- **TÜV SÜD:** TÜV SÜD is a well-known organization specializing in testing, inspection, and certification services for medical devices. With a strong emphasis on regulatory compliance and risk management, TÜV SÜD plays a vital role in ensuring the quality and safety of medical devices in the European market.
- **Bureau Veritas:** Bureau Veritas is a trusted name in the field of testing and certification services, including medical device testing. The company's expertise in quality assurance and compliance testing makes it a preferred choice for medical device manufacturers in Europe.
The Europe medical device testing market is witnessing significant growth due to the increasing focus on quality and safety standards for medical devices. With a diverse range of service types, sourcing options, and technologies available, market players are continuously innovating to meet the evolving needs of the industry.
The Europe medical device testing market is experiencing notable growth driven by the escalating emphasis on stringent quality and safety standards for medical devices. This growth is further propelled by the increasing complexity of medical devices, leading to a greater need for comprehensive testing services across the region. The market segmentation based on service type, sourcing type, and technology highlights the diverse landscape of the industry, catering to a broad spectrum of testing requirements for medical devices in Europe.
One of the key trends shaping the market is the rising demand for specialized testing services tailored to different categories of medical devices. As the regulatory landscape becomes more stringent, there is a growing need for testing services that address specific quality and safety parameters for various types of medical devices. This trend is driving market players to expand their service offerings and expertise in niche areas such as biological safety testing, chemical testing, and electrical safety testing to cater to the evolving needs of medical device manufacturers.
Moreover, the shift towards outsourced testing services is gaining traction in the Europe medical device testing market. Outsourcing testing activities to third-party service providers offers several advantages to medical device manufacturers, including access to specialized expertise, advanced testing technologies, and cost-effective solutions. This trend is expected to drive the growth of outsourced testing services in the region, with more companies opting for external partners to streamline their testing processes and enhance overall efficiency.
Another significant aspect influencing the market dynamics is the increasing adoption of advanced technologies for medical device testing. Technologies such as automation, artificial intelligence, and data analytics are revolutionizing the testing process, enabling faster, more accurate, and cost-effective evaluations of medical devices. Market players are investing in cutting-edge technologies to enhance their testing capabilities and deliver superior services to their clients, thereby staying ahead in a competitive market landscape.
Furthermore, the Europe medical device testing market is characterized by intense competition among key players vying for market share. Companies are focusing on strategic partnerships, collaborations, and acquisitions to strengthen their market position and expand their service offerings. The market is witnessing a trend towards consolidation, with larger players acquiring smaller firms to enhance their expertise and geographic reach in the region.
In conclusion, the Europe medical device testing market is poised for robust growth driven by the increasing demand for high-quality and safe medical devices. Market players are capitalizing on evolving trends such as specialized testing services, outsourced testing, advanced technologies, and strategic partnerships to stay competitive and meet the dynamic needs of the industry. As the regulatory environment continues to evolve, companies will need to adapt and innovate to maintain their relevance and leadership in the rapidly changing market landscape.The Europe medical device testing market is poised for substantial growth due to the increasing emphasis on stringent quality and safety standards within the medical device industry. As regulations become more stringent, the demand for comprehensive testing services continues to rise, creating opportunities for market players to innovate and deliver specialized solutions. One emerging trend shaping the market is the customization of testing services to cater to the specific requirements of different categories of medical devices. This trend reflects the industry's need for tailored testing methodologies to ensure compliance with varying quality and safety parameters across different types of medical devices.
Moreover, the shift towards outsourced testing services is gaining momentum, driven by the benefits offered to manufacturers seeking specialized expertise, advanced technologies, and cost-effective testing solutions. Outsourcing allows companies to streamline their testing processes and access a broader range of capabilities while maintaining a competitive edge in the market. This trend is expected to drive the growth of outsourced testing services as more companies recognize the advantages of partnering with external service providers.
The uptake of advanced technologies such as automation, artificial intelligence, and data analytics is revolutionizing the medical device testing landscape in Europe. These technologies enable faster, more accurate, and cost-effective evaluation of medical devices, enhancing the overall efficiency of testing processes. Market players are investing in cutting-edge technologies to strengthen their testing capabilities, improve service delivery, and differentiate themselves in the competitive market environment.
In the face of intense competition, key players in the Europe medical device testing market are focusing on strategic initiatives such as partnerships, collaborations, and acquisitions to expand their service offerings and consolidate their market position. This trend towards industry consolidation is driven by the quest for enhancing expertise, geographic reach, and overall competitiveness in the market. As companies continue to navigate dynamic regulatory environments and changing market demands, the ability to adapt, innovate, and forge strategic alliances will be critical to sustaining growth and leadership in the evolving Europe medical device testing market.
The Europe Medical Device Testing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/europe-medical-device-testing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Table of Contents:
- Europe Medical Device Testing Market Overview
- Economic Impact on Industry
- Competition by Manufacturers
- Production, Revenue (Value) by Region
- Supply (Production), Consumption, Export, Import by Regions
- Production, Revenue (Value), Price Trend by Type
- Market by Application
- Manufacturing Cost Analysis
- Industrial Chain, Sourcing Strategy and Downstream Buyers
- Europe Medical Device Testing Market Strategy Analysis, Distributors/Traders
- Europe Medical Device Testing Market Effect Factors Analysis
- Europe Medical Device Testing Market Forecast
- Appendix
Browse More Reports:
Global Cell Lysis and Dissociation Market
Global Polyethylene Packaging Market
Asia-Pacific Polyethylene Glycol Market
Global Non-Hodgkin Lymphoma Diagnostics Market
Global Rathke's Cleft Cyst Market
Global Methyl Acrylate Market
Asia-Pacific Head-up Display Market
Asia-Pacific Depression Screening Market
Global Smartphones Market
Asia-Pacific Ostomy Drainage Bags Market
Global Soyabean Meal Market
Global Intraocular Lens (IOL) Market
Middle East and Africa Underactive Bladder Market
Global Sixth Nerve Palsy Treatment Market
Global Bluetooth in Automotive Market
Global Organic Rice Flour Market
Asia-Pacific Magnetic Resonance Imaging Devices Market
Global Flavouring Agents Market
Global Oxygen Therapy Market
Global Anionic Surfactants Market
Middle East and Africa Viscosupplementation Market
Middle East and Africa Aerospace Adhesive - Sealants Market
Global Overlay Paper Market
Global Fanconi Anemia Treatment Market
U.S. Refrigerated Warehousing Market
North America Adhesive Tapes Market
Europe Aerospace Adhesive - Sealants Market
Global Allograft Market
Asia-Pacific Polylactic Acid (PLA) Market
Germany Wood Heating Stoves Market
Global Organic Cotton Market
Global Construction Punch List Software Market
Global Pen Needles Market
Global Myotonia Treatment Market
Asia-Pacific Laboratory Information Systems (LIS) Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness

