Pharmaceutical Manufacturing Software Market: How Automation and AI Are Enhancing Production Efficiency 2029
Global Pharmaceutical Manufacturing Software Market – A Deep-Dive Analysis (2023–2029)
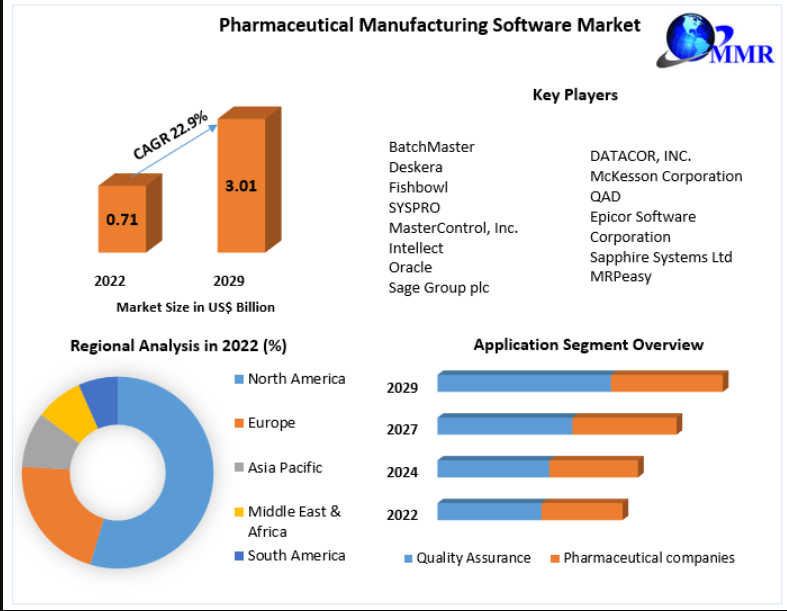
The Pharmaceutical Manufacturing Software Market, valued at US$ 0.71 billion in 2022, is on a rapid upward trajectory, projected to reach US$ 3.01 billion by 2029 at a robust CAGR of 22.9%. As pharmaceutical companies race toward higher efficiency, regulatory compliance, and faster innovation cycles, advanced software platforms are becoming indispensable pillars of modern drug manufacturing.
Market Overview
Pharmaceutical Manufacturing Software is designed to streamline, automate, and validate critical processes across manufacturing, quality control, documentation control, compliance management, and supply chain operations. These platforms manage SOPs, batch manufacturing records (BMRs), instrument calibration logs, validation protocols, and a broad range of regulated documents essential for cGMP compliance.
The COVID-19 pandemic served as a major accelerant for adoption. With global pressure to develop vaccines at record speed, pharmaceutical software solutions played a pivotal role in consolidating data, accelerating clinical operations, and stabilizing disrupted manufacturing networks. As a result, industry investments in digital infrastructure have grown exponentially.
To know the most attractive segments, click here for a free sample of the report:https://www.maximizemarketresearch.com/request-sample/78546/
Market Dynamics
- Drivers
➤ Digital Transformation Across Pharmaceutical Manufacturing
The global pharma sector is undergoing its most significant transformation in decades. Companies are replacing legacy systems with cloud-enabled, AI-powered, and highly integrated digital platforms. From formulation design to warehousing and distribution, digital infrastructure enables better decision-making, reduced downtime, and improved product quality.
➤ Shift Toward Agile, Cost-Efficient Manufacturing
Pharma organizations increasingly rely on ERP and MES (Manufacturing Execution Systems) to gain better visibility into operations. These platforms automate key functions:
- Manufacturing planning
- Procurement
- Formulation and batch scheduling
- Quality assurance and quality control
- Inventory and warehouse management
- Finance and compliance documentation
This automation enables companies to respond faster to demand fluctuations and maintain optimal operational efficiency.
➤ Growing Need for Regulatory Compliance
The pharmaceutical sector is one of the most heavily regulated industries in the world. Manufacturing software ensures:
✔ Data integrity
✔ 21 CFR Part 11 compliance
✔ Electronic signatures and audit trails
✔ Validation and quality management
✔ Real-time deviation and CAPA monitoring
As regulatory scrutiny intensifies, demand for validated and compliant software is set to rise.
- Restraints
➤ High Implementation Costs & Complex Integration
Many pharmaceutical manufacturers—especially small firms—hesitate to invest due to high upfront costs, complex integrations, and long customization cycles.
➤ Pricing Pressures and New Market Entrants
The sector faces intense cost pressures due to:
- Rapid generic competition
- Price regulations
- Reverse engineering of formulations
- New entrants and continuous innovation
This limits digital transformation budgets for some companies.
- Opportunities
➤ Rise of Virtual & Modular Manufacturing Facilities
Fast-track automation, simulation technologies, and digital twins allow companies to begin production system development while clinical trials are still underway.
➤ Emergence of Cloud-native, Scalable, and AI-driven Platforms
Cloud-based systems offer:
- Quicker deployment
- Lower upfront investment
- Remote monitoring
- Continuous software updates
- Real-time analytics
➤ Increasing Adoption in Clinical and Preclinical Trial Management
Platforms like eCRF, EDC, CTMS, and digital biomarker analytics are rapidly reshaping the clinical development process.
Market Segmentation
By Development Type
✔ Fast Track Automation (Dominant Segment)
This segment leads due to its ability to simulate processes, validate designs virtually, and shorten the overall time required for commercial drug development.
Key capabilities include:
- Process digital twins
- Modular manufacturing
- Advanced analytics
- Flexible I/O systems
✔ ERP Solutions
ERP systems ensure end-to-end visibility across procurement, manufacturing, finance, and quality control, making them essential for single or multi-facility manufacturers.
✔ Cloud-Based Platforms
Cloud solutions are gaining modernization momentum due to ease of maintenance, scalability and affordability.
By Application
- Quality Assurance
- Pharmaceutical Companies / Manufacturing Units
These platforms drive consistent quality output, reduce deviations, and enhance workforce productivity.
Large enterprises dominate adoption due to their multi-government compliance requirements and complex global supply chains.
To know the most attractive segments, click here for a free sample of the report:https://www.maximizemarketresearch.com/request-sample/78546/
Regional Insights
North America – Market Leader
North America commands the largest share owing to:
- Strong pharmaceutical manufacturing base
- High R&D investment
- Presence of major software vendors
- Early adoption of automation and cloud technologies
The U.S. continues to be a hub for pharma digitalization, with companies like ProcessPro expanding their cloud integration capabilities.
Europe – Strong Regulations, High Growth Potential
Europe’s strict compliance norms—EMA, GMP Annex 11, data integrity requirements—are driving widespread adoption of validated manufacturing platforms.
Asia Pacific – The Fastest Growing Market
Asia Pacific is witnessing an explosion in digital transformation efforts due to:
- Growing pharma manufacturing hubs in India, China, South Korea
- Government initiatives to digitalize healthcare
- Rising clinical trial activity
Increasing R&D spending—as outlined by WHO’s projection of global health expenditure—strengthens the region’s adoption outlook.
Competitive Landscape
The market is moderately fragmented, with a mix of global ERP vendors, MES solution providers, and specialized pharma software developers.
Key Players
- BatchMaster
- Deskera
- Fishbowl
- SYSPRO
- MasterControl, Inc.
- Intellect
- Oracle
- Sage Group plc
- DATACOR, INC.
- McKesson Corporation
- QAD
- Epicor Software Corporation
- Sapphire Systems Ltd
- MRPeasy
Leading players are investing heavily in AI-driven analytics, cloud-native architecture, real-time batch monitoring tools, and validation automation platforms.
Conclusion
The Pharmaceutical Manufacturing Software Market is entering a new era where digital ecosystems, AI-enhanced automation, and cloud-native platforms are becoming essential—not optional. The increasing complexity of drug development, heightened regulatory scrutiny, and the need for faster commercialisation are pushing pharma companies toward comprehensive software-driven manufacturing frameworks.
With high innovation speed and growing investment in digital pharma infrastructure, the industry is expected to witness a dramatic transformation through 2029.
- Pharmaceutical_Manufacturing_Software_Market
- Pharmaceutical_Manufacturing_Software_Market_Trend
- Pharmaceutical_Manufacturing_Software_Market_Insights
- Pharmaceutical_Manufacturing_Software_Market_Size
- Pharmaceutical_Manufacturing_Software_Market_Share
- Pharmaceutical_Manufacturing_Software_Market_Dynamics
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness

